Table of ContentsClose
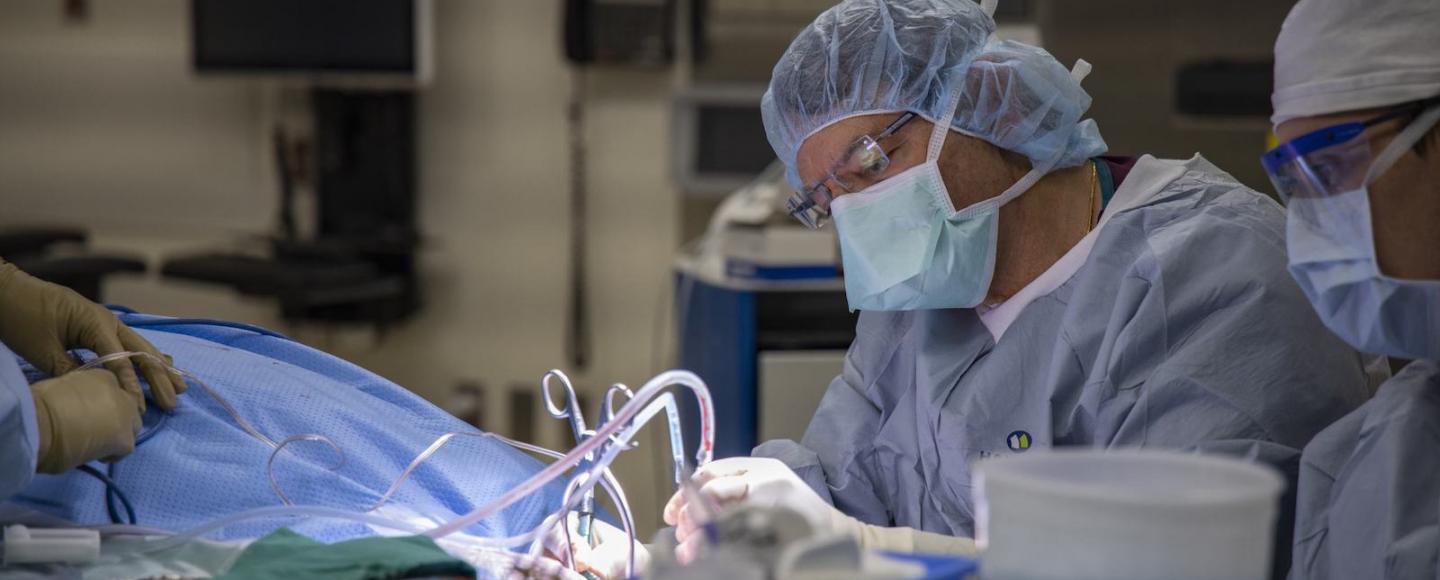
It takes a steady hand to successfully perform a cochlear implant procedure. Just ask Bruce Gantz (74MD, 80R, 80MS), who has completed thousands of these operations.
But even Gantz, one of the world’s top cochlear implant surgeons, admits he’s no match for the latest technology developed and tested at the University of Iowa.
For nearly 40 years, Gantz and his colleagues at the Iowa Cochlear Implant Clinical Research Center have worked to give their adult and pediatric patients the ability to experience the auditory world. Home to the only National Institutes of Health (NIH)-funded clinical research center for cochlear implants, the UI not only transforms the lives of patients but also fuels advancements in the evolution of a device that's been described as a modern miracle.
Last year, the UI center ushered in a new era in the field when Gantz performed the world's first robot-assisted cochlear implant electrode insertion. The robotic tool—recently approved by the Food and Drug Administration (FDA) in October and devised by fellow Iowa otolaryngology professor Marlan Hansen, MD (95R, 97F, 01R), and former resident physician Chris Kaufmann, MD, MS (17R)— could transform how surgeons perform cochlear implantations in the future.
Mitigating damage to preserve hearing
Gantz has trained a long line of future surgeons in the art of threading tiny electrode wires through the delicate cochlea. Among those former residents was Hansen, who joined the Carver College of Medicine faculty in 2003 and last year took over for Gantz as chair of the Department of Otolaryngology.
Of their many key research findings, Hansen and Gantz have proven that the slower those electrodes are pushed through the cochlea, the less trauma occurs to the high-risk sections of the inner ear.
“The inner ear has a lining that is very reactive to damage, it’s only a few cells thick. And we don’t have any haptic control [technology that simulates the sense of touch] when we’re completing these procedures. There is no sensation that we’re up against the edges or we’re causing any issues,” says Gantz, UI professor of otolaryngology and neurosurgery.
Studies show that between 15% to 50% of implant recipients may experience an additional loss of their natural hearing after surgery. By mitigating the damage caused by the electrode scraping against the walls of the cochlea, more functionality can be preserved in patients who have residual hearing.
"What Bruce and the team at Iowa have demonstrated is that any acoustic hearing you can save is really helpful for patients, especially to hear complex sounds, music, and in noisy situations," Hansen says. "It helps their brain process sound, and more importantly, they don't need as much cognitive effort to hear and understand."
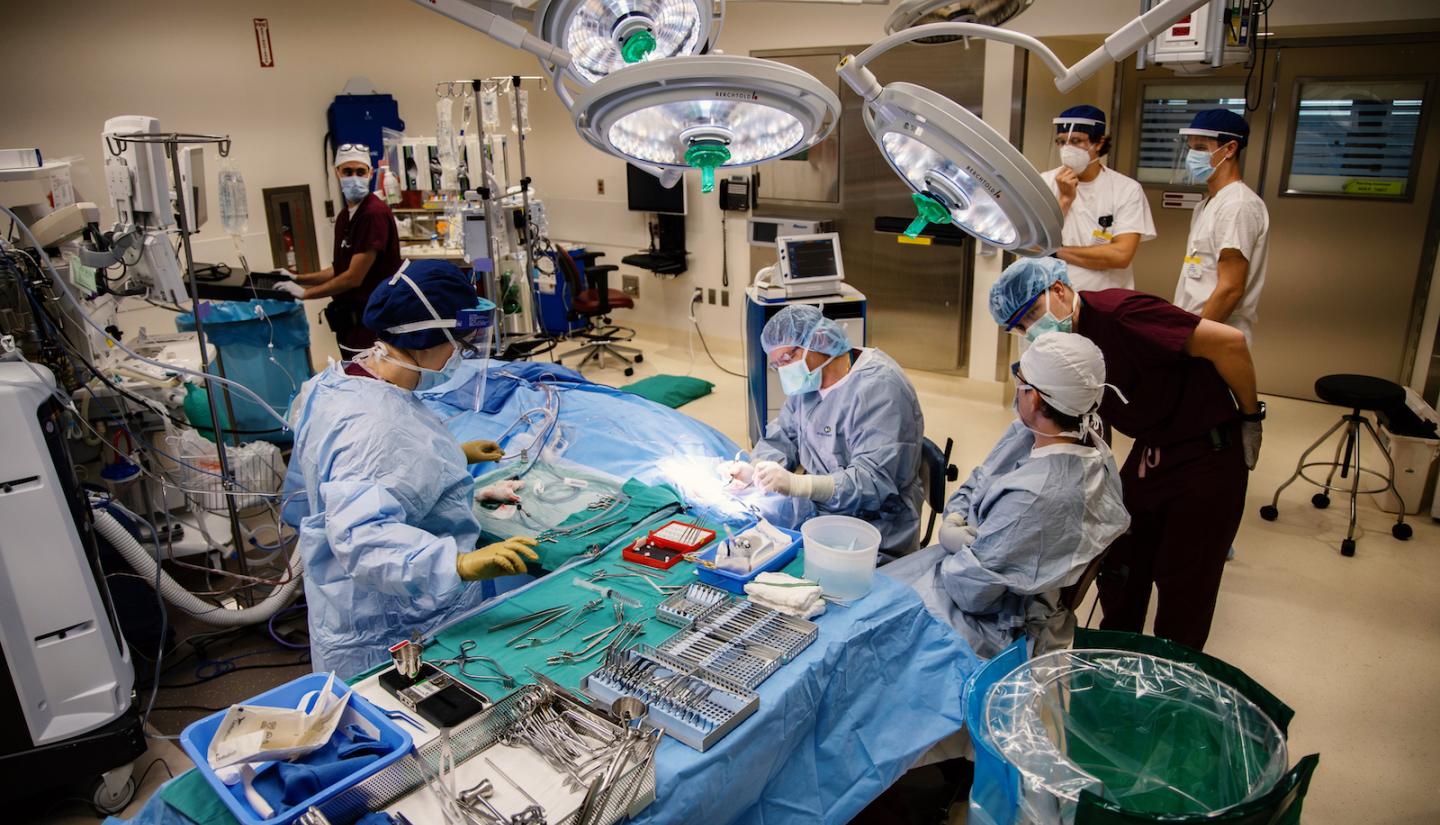
With that goal in mind, Hansen and Kaufmann, a former resident in his lab with a background in biomedical engineering, collaborated on a project to standardize electrode insertion and make the maneuver more precise. Their solution was the creation of a small robotic device to help guide the electrode through the spiraling cochlea, and in 2015 they founded a spin-out company called iotaMotion to develop the tool, named iotaSoft.
The recent FDA approval of the device will help the team cross a major hurdle in bringing their product to the market after years of research and development.
Today, the privately held company has grown into a nine-person operation based in Iowa City, with Hansen serving as chief medical officer and Kaufmann serving as president and leading research and development. The company has raised more than $4.5 million in private financing, and it has received more than $4 million in grant support from the NIH and the National Science Foundation.
During cochlear implant surgery, the cigar-sized robotic device is affixed to a patient's head with small screws. The surgeon uses a fine, curved tool to help position the electrodes through the incision behind the ear, then presses a foot pedal that begins the robot's slow, motorized insertion process. At a rate of just 0.1 millimeters per second, the robot nudges the electrode into the inner ear with a gentle precision that surpasses even the most steady-handed surgeon.
"My hand, even though I've done this maybe 3,000 times or more, is not as good as a micromotor," says Gantz. "Electrodes that are the least damaging are very thin, so it's like putting a wet piece of spaghetti in a tube. What the robot does is stabilizes it."

The iotaSoft
This small robotic device is used to help guide the electrode through the spiraling cochlea during a cochlear implant procedure. At a rate of just 0.1 millimeters per second, the robot nudges the electrode into the inner ear with a gentle precision that surpasses even the most steady-handed surgeon.
Last fall, Gantz became the first doctor in the world to perform a robot-assisted cochlear implant surgery during a clinical trial at Iowa for iotaMotion's device. Since then, Gantz has used it to successfully guide more than 15 cochlear implant procedures.
The device is one of several new technologies in the works at iotaMotion, which is also developing a robot-assisted control system that allows clinicians to reposition previously implanted electrodes to account for new hearing loss throughout the lifetime of their patients without the need for additional surgery.
Helping those with residual hearing
While cochlear implants have traditionally been used to treat patients with complete hearing loss, patients with moderate hearing loss have increasingly turned to the devices in recent years. Iowa was one of the first institutions to start expanding the criteria for implantation to include those with residual hearing. Gantz says he’s seen more patients with low-frequency residual hearing over the past decade, and now roughly 40% of his patients have useful residual hearing.
6.3 million people in the U.S. affected by moderate hearing loss.
And with as many as 6.3 million people in the U.S. affected by moderate hearing loss, Hansen and iotaMotion see a growing market that could benefit from technology that preserves residual hearing.
“We have some patient groups in which 75% to 80% still have residual hearing after 15 years with the implant in place,” Gantz says. “If we get the cochlear implant in place and don’t damage the inner ear, the chance of the device being useful for long periods of time is quite promising.”
Iowa Cochlear Implant Clinical Research Center
Home to the only National Institutes of Health (NIH)-funded clinical research center for cochlear implants, the UI not only transforms the lives of patients with hearing loss but also fuels advancements in the field.
