Table of ContentsClose
News briefs
For the 33rd year in a row, University of Iowa Hospitals & Clinics has been recognized as the only hospital in Iowa to achieve a national ranking by U.S. News & World Report.
The 2022-2023 U.S. News “Best Hospitals” rankings also list eight adult care specialties at UI Hospitals & Clinics within the top 10% of programs nationally, identified as “high-performing.”
-
Cancer: High-Performing
-
Ear, nose, and throat: No. 31
-
Gastroenterology and GI surgery: High-Performing
-
Geriatrics: High-Performing
-
Ophthalmology: No. 7
-
Orthopedics: High-Performing
-
Pulmonary and lung surgery: High-Performing
-
Urology: High-Performing
Additionally, UI Stead Family Children’s Hospital—Iowa’s only nationally ranked children’s hospital—is listed among the nation’s best in seven specialties in the 2022-23 “Best Children’s Hospitals” rankings.
-
Neonatology: No. 26
-
Pediatric cancer: No. 38
-
Pediatric nephrology: No. 27
-
Pediatric orthopedics: No. 36
-
Pediatric pulmonology: No. 50

Michael J. Welsh (74MD, 77R) has won the 2022 Shaw Prize in Life Science and Medicine with Paul A. Negulescu, senior vice president and site head for San Diego research with Vertex Pharmaceuticals Inc.
The prize was awarded for landmark discoveries of the molecular, biochemical, and functional defects underlying cystic fibrosis (CF) and the identification and development of medicines that reverse those defects and can treat most people affected by this genetic disorder.
The Shaw Prize is considered one of the most internationally prestigious awards in science and its application, and it carries a shared monetary award of $1.2 million. It honors individuals who have made “discoveries in the biomedical sciences and innovations in clinical medicine that have led to significant victories in our longstanding war against illness and suffering.”
Welsh is a professor in the UI Department of Internal Medicine and its pulmonary, critical care, and occupational medicine division. He has been leading groups of scientists studying lung biology and CF for 40 years.
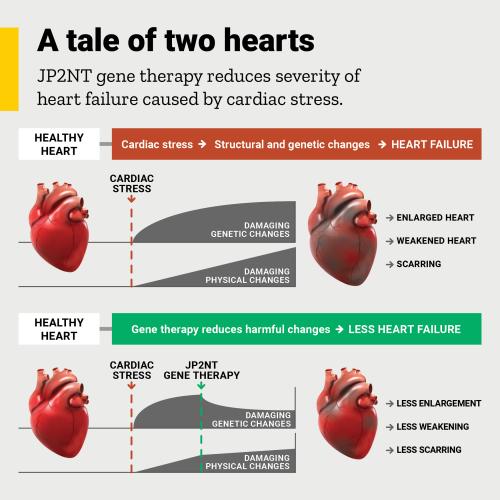
Four years ago, University of Iowa scientist Long-Sheng Song, MD, made an unexpected discovery that he thought might form the basis for a new gene therapy treatment for heart failure. In his latest study, he shows that the approach works in mice, protecting the animals from heart failure due to cardiac stress.
Heart failure is a leading cause of death and illness worldwide, affecting more than 26 million people. Cardiac stress—from high blood pressure, blocked arteries, or heart attack—is one cause of heart failure.
In an earlier study, Song and his team found that during cardiac stress, a protein known as junctophilin-2 (JP2) is cleaved into two pieces, and one of those fragments (JP2NT) can turn off the expression of certain damaging genes in heart cells. He speculated that harnessing this self-protecting mechanism might lead to a new treatment for heart failure.
In the new study, published in the journal Circulation Research, Song’s team shows that delivering the JP2NT fragment to heart cells using an adeno-associated, virus-mediated gene delivery method can limit the development of heart failure when it is given either before or after the onset of cardiac stress.
The researchers plan to further test this gene therapy approach in large animal models and, if the results are promising, pursue a clinical trial in patients with heart failure.

Gene therapy repaired severely damaged muscle and significantly improved the survival of mice with a particular type of muscular dystrophy, according to a study from Carver College of Medicine researchers.
“Showing that we can rescue muscles already damaged by the disease is an important proof of concept, because most patients with this type of muscular dystrophy are not diagnosed until after onset of muscle degeneration, so any successful therapy would have to be able to repair that damage,” says Kevin Campbell, PhD, UI professor and chair of molecular physiology and biophysics and senior author of the study, which was published in Science Advances.
Much of Campbell’s research has focused on a type of muscular dystrophy known as dystroglycanopathies, which are caused by genetic mutations that damage the function of a critical muscle protein called alpha-dystroglycan. This muscle protein acts as a structural bridge between muscle cells and their surrounding tissue. Specifically, the mutations disrupt the formation of a long sugar molecule called matriglycan, which is responsible for stabilizing muscle’s structural integrity.
Previous work by Campbell’s team found that a protein, known as LARGE, is critical for building the matriglycan chains. Mice that are missing the LARGE protein do not make matriglycan chains and are good models for dystroglycanopathies. As these mice age, their muscles deteriorate, they lose weight, and they have shorter lifespans than wild-type mice. The researchers previously showed that overexpressing LARGE can rescue muscle function in mice with dystroglycanopathies.
In the new study, Campbell and his colleagues used the same genetically engineered mice but allowed them to age and develop advanced muscle disease before treating some of them with gene therapy that delivered the LARGE protein back into the muscle cells. The treated mice recovered muscle strength and function, which led to improved mobility and respiratory function and normalized metabolism. All but one of the treated mice lived to at least 65 weeks of age, compared to only 50% of the untreated mice surviving to 45 weeks of age.
Using techniques borrowed from the culinary arts to control dosage and delivery, a new study demonstrates carbon monoxide-containing materials as a safe and effective therapy for inflammatory diseases.
Carbon monoxide is more commonly known as a colorless, odorless, and potentially deadly gas. But evidence from animal studies and clinical trials suggests that low doses of carbon monoxide might help treat inflammation in a variety of diseases. However, ensuring the safety of inhaled doses presents a major challenge to widespread use of this gas in patients.
This challenge led a team of scientists from MIT, Brigham and Women’s Hospital, the University of Iowa, and Beth Israel Deaconess Medical Center to seek a different delivery method through the gastrointestinal (GI) tract.
"Just as molecular gastronomy has expanded the physical composition of foods, we used those same techniques to incorporate carbon monoxide into materials, such as foams, that can be safely delivered to the GI tract,” says James Byrne, MD, PhD, UI assistant professor of radiation oncology and biomedical engineering, and one of the lead authors on the study.
In the new study, the research team developed and tested carbon monoxide-containing materials in mice, rats, and pigs. They found that it showed benefits in three different inflammatory conditions without causing any dangerous side effects.
Delivering the foam rectally reduced inflammation of the colon in a model of inflammatory bowel disease and reduced inflammation of the rectum caused by radiation treatment.
Additionally, the researchers also showed that foam delivered to the rectum also reversed acute liver failure caused by acetaminophen overdose.
The findings were published June 29 in Science Translational Medicine.
About 10.5% of U.S. households are food insecure. But when it comes to pregnant women, that number jumps to nearly 20%.
Food insecurity has been linked to an increased risk of pregnancy complications, such as high gestational weight gain, gestational diabetes, high blood pressure, preterm birth, and postpartum depression. It also complicates managing preexisting health conditions, such as diabetes.
To help support patients who are facing challenges outside the clinical setting, Craig Syrop, MD, professor emeritus in the Department of Obstetrics and Gynecology, his colleagues, and a group of interdisciplinary undergraduate and graduate students began the Upstream Initiative.
“Social determinants of health, such as food insecurity, absolutely shape disparities in health outcomes,” Syrop says. “People may not know that 80% of health outcomes lie outside clinical interventions. We like to think that the care we provide in clinics makes a big difference—and we do make a big difference—but if you’re going to really help people change their lives, you have to also attend to those other aspects: food, housing, transportation, finances, those sorts of things.”
Patients who visit the High-Risk Obstetric Clinic answer questions about food insecurity and transportation needs as part of a standard intake questionnaire. If patients indicate they face one of those needs, an Upstream volunteer meets with them to discuss potential resources. This includes discussing eligibility requirements for federally funded health and nutrition programs, as well as resources in their local communities, such as food pantries, which the volunteers identify with help from Iowa Compass and 211 Iowa databases.
Between January 2018 and December 2021, the Upstream Initiative completed 6,143 screenings spanning 2,402 pregnancies. Of those, 684 screenings were positive for food insecurity. On average every year, they serve patients from 67 counties in Iowa.
Additionally, the program in February received $100,000 from the UI Carver College of Medicine to partner with the Hawkeye Area Community Action Program to provide food resources on the spot to pregnant patients with food insecurity.
“This won’t be a food bank where you come in anytime,” says UI College of Public Health student Johanna Knutson. “Instead, we’ll be able to give them a food box to take home that will last a few days, and it will sort of be a bridge between the appointment and hopefully using local resources we help connect them with.”
Knutson says other projects that are in very early stages involve providing diapers and menstrual products to patients and developing a partnership to provide patients legal advice, particularly around housing issues.
In the news





Paper trail
Watterberg KL, Walsh MC, Li L, Chawla S, D'Angio CT, Goldberg RN, Hintz SR, Laughon MM, Yoder BA, Kennedy KA, McDavid GE, Backstrom-Lacy C, Das A, Crawford MM, Keszler M, Sokol GM, Poindexter BB, Ambalavanan N, Hibbs AM, Truog WE, Schmidt B, Wyckoff MH, Khan AM, Garg M, Chess PR, Reynolds AM, Moallem M, Bell EF, Meyer LR, Patel RM, Van Meurs KP, Cotten CM, McGowan EC, Hines AC, Merhar S, Peralta-Carcelen M, Wilson-Costello DE, Kilbride HW, DeMauro SB, Heyne RJ, Mosquera RA, Natarajan G, Purdy IB, Lowe JR, Maitre NL, Harmon HM, Hogden LA, Adams-Chapman I, Winter S, Malcolm WF, Higgins RD; Eunice Kennedy Shriver NICHD Neonatal Research Network. Hydrocortisone to Improve Survival without Bronchopulmonary Dysplasia. N Engl J Med. 2022 Mar 24;386(12):1121-1131. doi: 10.1056/NEJMoa2114897. PMID: 35320643; PMCID: PMC9107291.
Hipp HS, Crawford S, Boulet S, Toner J, Sparks AAE, Kawwass JF. Trends and Outcomes for Preimplantation Genetic Testing in the United States, 2014-2018. JAMA. 2022 Apr 5;327(13):1288-1290. doi: 10.1001/jama.2022.1892. PMID: 35380591; PMCID: PMC8984775.
Scherer AM, Parker AM, Gidengil CA, Gedlinske AM, Askelson NM, Petersen CA, Lindley MC. COVID-19 Vaccine Uptake and Intentions Following US Food and Drug Administration Approval of the Pfizer-BioNTech COVID-19 Vaccine. JAMA Intern Med. 2022 Jun 1;182(6):678-680. doi: 10.1001/jamainternmed.2022.0761. PMID: 35404416; PMCID: PMC9002709.
Russell SR, Drack AV, Cideciyan AV, Jacobson SG, Leroy BP, Van Cauwenbergh C, Ho AC, Dumitrescu AV, Han IC, Martin M, Pfeifer WL, Sohn EH, Walshire J, Garafalo AV, Krishnan AK, Powers CA, Sumaroka A, Roman AJ, Vanhonsebrouck E, Jones E, Nerinckx F, De Zaeytijd J, Collin RWJ, Hoyng C, Adamson P, Cheetham ME, Schwartz MR, den Hollander W, Asmus F, Platenburg G, Rodman D, Girach A. Intravitreal antisense oligonucleotide sepofarsen in Leber congenital amaurosis type 10: a phase 1b/2 trial. Nat Med.2022 May;28(5):1014-1021. doi: 10.1038/s41591-022-01755-w. Epub 2022 Apr 4. PMID: 35379979; PMCID: PMC9117145.
Wong LR, Zheng J, Wilhelmsen K, Li K, Ortiz ME, Schnicker NJ, Thurman A, Pezzulo AA, Szachowicz PJ, Li P, Pan R, Klumpp K, Aswad F, Rebo J, Narumiya S, Murakami M, Zuniga S, Sola I, Enjuanes L, Meyerholz DK, Fortney K, McCray PB Jr, Perlman S. Eicosanoid signalling blockade protects middle-aged mice from severe COVID-19. Nature. 2022 May;605(7908):146-151. doi: 10.1038/s41586-022-04630-3. Epub 2022 Mar 21. PMID: 35314834.
