Table of ContentsClose
Theranostics: Revolutionizing cancer care
Legacy of imaging technology advancements helps unlock new treatment options
Theranostics—a pioneering fusion of therapeutic and diagnostic technologies—is redefining the cancer treatment landscape, with University of Iowa Health Care blazing a path forward.
“We know that radiation is probably the single most effective agent at treating cancers,” says John Buatti, MD, professor in the Carver College of Medicine Department of Radiation Oncology. “While radiation can selectively damage cancer cells more than normal tissues, it can also damage normal tissues. With theranostics, we've been able to use precision medicine to link radiation to molecular targets—delivering radiation more precisely to tumors without harming surrounding cells.”
Founded on a legacy of advancements in imaging technology, UI Health Care’s current theranostics program is the product of a decades-long commitment to interdisciplinary collaboration—bridging radiology, radiation oncology, and nuclear medicine.
With more than a dozen active and pending theranostics trials, UI researchers are helping to not just advance cancer research and understanding but provide new options for patients who otherwise haven’t had access to effective treatments.

Origins of theranostics
Theranostics’ roots trace back to the early 1940s, when radioiodine was initially used to treat hyperthyroidism. UI Health Care spearheaded the adoption of innovative imaging technologies and was among the first in the nation to receive a computerized tomography (CT) scanner.
In the 1990s, nuclear medicine specialist Richard Hichwa, PhD, led efforts to build the UI’s first cyclotron and positron emission tomography (PET) scanner. This made Iowa a frontrunner in PET imaging techniques, especially in harnessing radioactive isotopes and using radiochemistry to visualize and diagnose physiological processes, specifically cancers. As a result, UI Health Care became a leading PET imaging center in the United States.
Imaging expertise would eventually “pave the way for developing the first cancer-targeted theranostic molecules and subsequent clinical trials,” says Yusuf Menda, MD (00R, 01F), a professor in the UI Department of Radiology and director of its nuclear medicine division. Menda also is director of a Society of Nuclear Medicine & Molecular Imaging-certified Radiopharmaceutical Therapy Center of Excellence at Iowa and co-leader of the UI Holden Comprehensive Cancer Center Free Radical Metabolism and Imaging Program.
In the early 2000s, UI researchers M. Sue O’Dorisio, MD, PhD; Thomas O'Dorisio, MD; and David Bushnell, MD, spearheaded clinical trials evaluating the first theranostic compounds for neuroendocrine tumors (NETs). For their work, the Iowa team—members of UI Holden Comprehensive Cancer Center—received a Specialized Program of Research Excellence (SPORE) grant from the National Cancer Institute. It was the first SPORE grant ever awarded to fund research on NETs.
UI Health Care researchers also participated in the pivotal phase 3 clinical trials that led to the approval of Lutathera for neuroendocrine tumors and Pluvicto for prostate cancer. The UI also leads the efforts to establish dosimetry-based personalized treatment administration.
Trial runs
348: Average annual number of UI Health Care clinical trials from 2018-2022
1,337: Average annual number of participants enrolled in UI Health Care clinical trials from 2018-2022
300+: Patients have been enrolled in UI Health Care theranostics trials
10: Number of active UI Health Care theranostics clinical trials
5: Number of pending UI Health Care theranostics clinical trials
Personalized cancer therapies
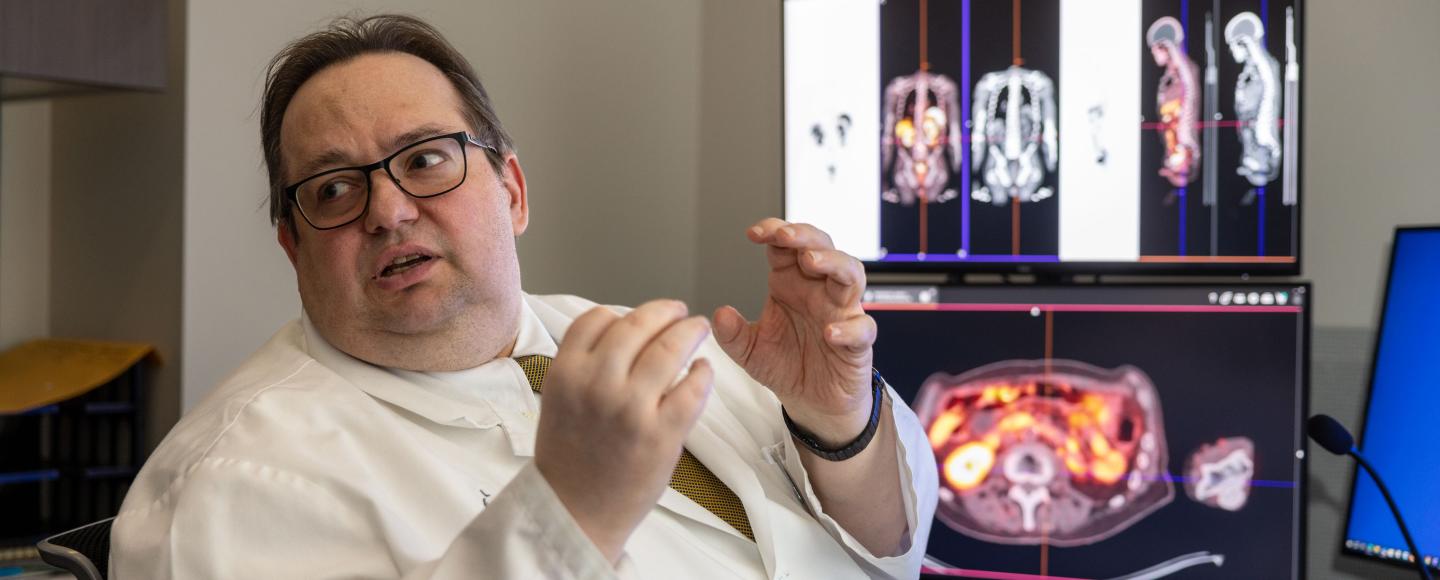
Theranostics revolves around leveraging nuclear medicine’s quantitative imaging expertise and radiation oncology’s treatment delivery prowess. In general, the big difference between traditional cancer imaging techniques and theranostics is that theranostics gives physicians the ability to see and target specific receptors on cancerous cells.
The theranostics process begins when specific transport molecules with attached positron emitters, most commonly gallium, are injected into a cancer patient’s bloodstream. These molecules target specific receptors located on the outside of cancer cells, giving doctors a precise visualization of the tumors on PET scans. A second injection delivers the same transport molecule and a different therapeutic beta emitter (most commonly lutetium) to cancer cells, providing lethal doses of radiation at a cellular level with minimal impact on surrounding healthy tissue.
The effect of this theranostics visualization process is twofold:
- Physicians can use drugs to only target cancer cells, leaving noncancerous cells unharmed.
- Physicians can calculate the most appropriate radiation dose for the patient based on the imaging, which limits side effects and can improve treatment results, especially for metastatic cancers.
For instance, less radiation would be delivered if a patient has fewer receptors on their cancer cells. Ultimately, this means each patient receives a different dose of a therapeutic drug depending on the number and configuration of their receptors.
Pioneering Iowa research using alpha emitters
For most of the 2000s, theranostics for cancer at UI was limited to neuroendocrine cancer treatments. Then, in 2022, UI Health Care specialists participated in the first national clinical trial that evaluated a therapeutic drug for prostate cancer. This marked the first non-neuroendocrine theranostics treatment and a major step forward, as prostate cancers are much more common than neuroendocrine cancers. The Iowa team is now overseeing theranostics trials for additional cancers, including pancreatic cancer.
Innovation goes beyond just the type of cancer that’s being researched in the UI theranostics lab and extends to the fundamental characteristics of these therapeutic agents.
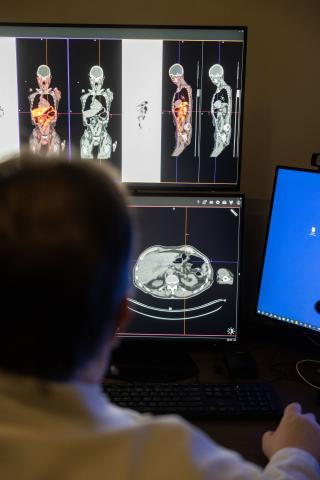
“Part of our current research efforts focuses on using alpha emitters instead of beta emitters to deliver radiation to tumors,” Menda says. “We expect that using alpha particles will improve tumor treatment compared to the current standard approved isotopes because alpha emitters are expected to be significantly more effective in killing tumors.”
UI Health Care is actively recruiting for an alpha-emitting study in a phase 1 trial and has imaged 10 patients in preparation for the alpha emitter therapy. The team is waiting on toxicity data before moving to the next trial cohort.
“The imaging prior to treatment provides important data about the radiation doses and bio-distribution, or how it spreads throughout the body, of the agent,” Menda says.
UI physicists Mark Madsen, PhD (retired), and Stephen Graves, PhD, assistant professor in the Department of Radiology and its nuclear medicine division, have been instrumental in helping the UI team use the imaging information to calculate dose measurements. This data helps personalize patient treatment, maximizing dose safety and efficacy.
“If you think about it,” Buatti says, “it's complicated to figure out how much radiation dose gets to an actual tumor. If I have some form of radioactivity, and I inject it into your blood, and you have this molecular uptake at a tumor, it almost always has some of the radioactive compound metabolized. Maybe some gets excreted in the urine, maybe it gets excreted in other ways, but 100% of it doesn't just automatically go to the tumor and stay there.”
Study groups
UI Health Care leads hundreds of clinical trials annually for various medical conditions, with researchers evaluating therapies and interventions to redefine treatment for current and future patients.
The prevailing question, Buatti poses, is when you give someone an injection like this, how much is the tumor getting over the time that the radioactivity is present?
“We've worked on developing deep-learning-based algorithms to be able to automatically identify normal organs and bone marrow as well as multiple tumors very quickly so that we can calculate doses more accurately,” he says.
The future of cancer care
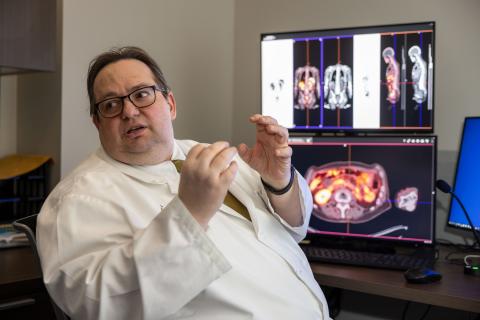
Researchers are examining a potential new cancer receptor target known as fibroblast activating protein inhibitor (FAPI). Iowa is one of four sites in this phase 1 trial, which could impact how solid tumors, like pancreatic cancer or sarcomas, are identified and targeted by theranostic products. Patients may qualify for theranostic treatment if tumors have specific FAPI receptors.
The UI team is also looking at compounds being tested for small-cell lung cancer, melanoma, and breast cancer.
“From a patient care point of view, these compounds are for people who don't have good, effective treatment options,” Buatti says. "They're not good candidates for surgery. They're not good candidates for standard radiation therapy. They're not good candidates for chemotherapy. Theranostics treatments fill a real void."
