Table of ContentsClose


James Byrne appreciates a well-made cappuccino, but not because he’s a connoisseur of espresso drinks.
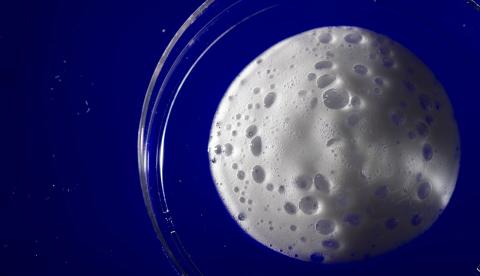
He’s more interested in the foam.
For Byrne, MD, PhD, an assistant professor in the University of Iowa Carver College of Medicine Department of Radiation Oncology, it’s using foam—and creams, hydrogels, and solids—to create new, biocompatible materials that may improve the effectiveness of chemotherapy and radiation in treating cancers. Specifically, these gas-entrapping materials, or GeMs, are designed to carry high concentrations of therapeutic gases directly into tissues, including tumors.
In a study published last year in the journal Advanced Science, Iowa researchers led by Byrne and assistant research scientist Jianling Bi, PhD, report using GeMs to deliver high levels of oxygen directly into tumors, which boosted chemotherapy and radiation therapies in mouse models of prostate cancer and a type of sarcoma.
“We’ve known for a long time that if you increase the amount of oxygen within a tumor, you can make it more responsive to radiation, certain chemotherapies, and potentially immunotherapies,” says Byrne, a member of Holden Comprehensive Cancer Center at Iowa. “The challenge has been how to deliver an effective dose of oxygen in a safe, controlled fashion.”
The research team—which includes colleagues from the Massachusetts Institute of Technology, Beth Israel Deaconess Medical Center, and Harvard Medical School—showed that GeMs can increase oxygen levels in solid tumors, rendering them more vulnerable to chemotherapy or radiation. The increased oxygen levels also appeared to improve immune reactivity, which is key to generating a response to immunotherapy.
Taking a page from the cookbook
In the culinary arts, foams and foam variations are not uncommon—think of whipped cream, lemon meringue, and chocolate mousse.
Byrne, Bi, and colleagues drew inspiration from molecular gastronomy—the scientific focus on the chemical processes involved in cooking—to create GeMs comprising three primary ingredients: a gas, a foaming agent, and a thickening agent.
“We use several unique, custom-built pressurized systems to incorporate high concentrations of gas into small volumes of these biocompatible materials, which can be injected or implanted into tissues and allow for prolonged, controlled release of the gas,” Byrne says.
The foam GeMs, for example, are created using a whipping siphon—essentially the same device baristas use—but reverse-engineered to accept various gases, including oxygen. The lab’s whipping siphons use safe, low-cost components found in many processed foods to make the GeMs.
By varying the quantity of each component, the researchers can control the release of oxygen from the material. And because the GeMs are manufactured with safe and edible components, Byrne notes that the translatability of these materials for cancer care is likely to be extremely high.
The ability to implant or inject GeMs directly into the tumor is another advantage. Intra-tumoral delivery of cancer treatment is an approach that has blossomed over the past decade due to the ability to place high concentrations of drugs inside the tumor with minimal side effects. Foam, in particular, can be injected into areas of a tumor that are harder to treat or remove by surgery.
Carbon monoxide-infused foam boosts anti-cancer activity
Oxygen is not the only gas molecule with cancer-fighting potential. Previous lab research by Byrne and colleagues found that very low doses of carbon monoxide may help treat inflammation in a variety of diseases—and may benefit cancer treatments, as well.
Researchers have known that autophagy—a cell’s natural recycling system—is increased in cancer cells compared to healthy cells, suggesting that inhibiting autophagy may be a way to target cancer cells. However, results from numerous clinical trials testing autophagy inhibitors have been inconclusive, with little or no benefit for some patients.
Searching for insight into why autophagy inhibition only seems to work some of the time, Byrne and colleagues made the discovery that smokers in two of the previous trials of autophagy inhibitors seemed to do better than non-smokers.
This finding was important, since smoking is also associated with increased levels of carbon monoxide, a gas molecule that can increase autophagy in cells in a way that could enhance the anti-cancer effect of autophagy inhibitors. The key was to “harness that benefit and take it into a therapeutic platform,” Byrne says.

With the GeMs, the research team already had a platform to test their ideas. For this study, the researchers created a drinkable foam infused with carbon monoxide. When lab mice with pancreatic and prostate cancers were fed the carbon monoxide foam and simultaneously treated with an autophagy inhibitor, tumor growth and progression was significantly reduced in the animals.
The multicenter research team—comprising scientists from MIT, Harvard, the University of Pennsylvania, Rutgers Cancer Institute, the University of North Carolina Wilmington, and Oregon Health and Science University—also showed that combining carbon monoxide with autophagy inhibitors had a significant anti-cancer effect in human prostate, lung, and pancreatic cancer cells in petri dishes.
The study results—also published in Advanced Science—support the idea that “safe, therapeutic levels of carbon monoxide, which we can deliver using GeMs, can increase the anti-cancer activity of autophagy inhibitors, opening a promising new approach that might improve therapies for many different cancers,” Byrne says.
Researchers look to clinical trials
The next step is to test GeMs in clinical studies. Byrne hopes to begin a phase 1 clinical trial of oxygen-infused GeMs in sarcoma patients in 2025. He and his research colleagues are in the process of filing an investigational new drug (IND) application with the U.S. Food and Drug Administration.
CO-infused foam and diabetes wound care
James Byrne, MD, PhD, and colleagues have developed a carbon monoxide-infused foam that can be applied topically and improve healing in models of diabetes-related skin wounds and pressure ulcers.
In the study—published in March in the journal Device—Byrne and his colleagues first showed that exposure to carbon monoxide (CO) enhances migration of human skin cells in a petri dish, a characteristic that could help the wound-healing process. They then developed a foam made from hyaluronic acid—a substance used in many skincare products—infused with carbon monoxide. The foam can be applied safely to wounds and allows for prolonged delivery of the gas directly at the wound site for therapy. The gas-entrapping material also contained silver nanoparticles, which have antimicrobial properties and have been shown to promote wound healing.
Using mouse models of diabetic wounds, the researchers showed that the CO-infused foam promoted healing responses in deep skin wounds and pressure ulcers when compared to a foam infused with nitrogen, which does not have the same immune boosting properties as carbon monoxide, or when compared to untreated wounds.